SuppCo set out to make sense of supplements and it’s delivering. After testing 10 NAD+ products, half failed. These are the ones worth keeping in your cart
If you’ve been paying attention to the longevity space, you’ve likely noticed NAD+ everywhere: in capsules, IV drips and a topic of chatter among the 40-plus crowd. But a new analysis found that half of the most popular NAD+ supplements contained minimal or no NAD+ at all.
So finds SuppCo, a supplement transparency and stack management platform that is taking aim at the supplement industry’s quality-control problem through independent, third-party lab testing. The findings are part of the startup’s second installment of its “Tested” series, which anonymously purchases supplements on Amazon and sends them to ISO 17025-certified labs for analysis.
And in the high-priced quest for longevity, where consumers shell out for products promising to extend healthspan, SuppCo’s latest results expose quality gaps that could save buyers money and spare them false hope.
It’s also not the first time the company, which raised $5.5 million last year in a seed round, has caught popular products falling short. In its recent creatine report, SuppCo revealed that some of Amazon’s top-selling creatine supplements contained no creatine at all, a finding it bluntly called “massive failures.”
NAD⁺ Products Take Their Turn in the Hot Seat
As SuppCo noted in its report, it chose to test capsule supplements marketed as containing nicotinamide adenine dinucleotide (NAD+) itself, stating that trending, poorly understood ingredients are a common source of quality issues. An even bigger catch? Simply swallowing an NAD+ labeled supplement may not do much at all.
That’s because the coenzyme is notoriously hard to absorb in its intact form, with little evidence that it reaches the bloodstream or cells directly. Most researchers (and many high-quality commercial products) point instead to precursors like nicotinamide riboside (NR, a form of vitamin B3) and nicotinamide mononucleotide (NMN), which are more readily absorbed and have been shown to raise NAD⁺ levels in humans, according to SuppCo.
SuppCo’s latest investigation targeted 10 of the most-used NAD+ supplements stacked on its platform, all labeled as containing nicotinamide adenine dinucleotide itself, not precursors like nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN).
Each product was purchased anonymously from Amazon, always from the brand’s official storefront, never third-party resellers. SuppCo documented every order with lot numbers and screenshots, and brands were never notified or allowed to send samples directly. Testing then took place at an ISO 17025-certified laboratory, and products that failed an initial analysis were sent back for confirmatory testing.
Three metrics drove the evaluation:
- NAD+ per serving: Pass/fail based on delivering at least 95% of the labeled amount
- Percent of label claim: The actual NAD+ content compared with the stated amount on the label
- Heavy metals: Levels of lead, mercury, cadmium and arsenic, measured against California Proposition 65 standards
Five of Ten NAD⁺ Supplements Fail to Deliver
The findings were stark: five products failed both tests, in some cases with almost no detectable NAD+ (Maripolio Liposomal NAD+). Notably, most of the underperformers ranked among SuppCo’s top 10 most-used NAD⁺ supplements.
One high-volume seller, however, was an exception. It narrowly failed on the first pass but cleared the second, while triggering what SuppCo called a “caution” flag over labeling that could leave buyers unclear about their actual dosage.
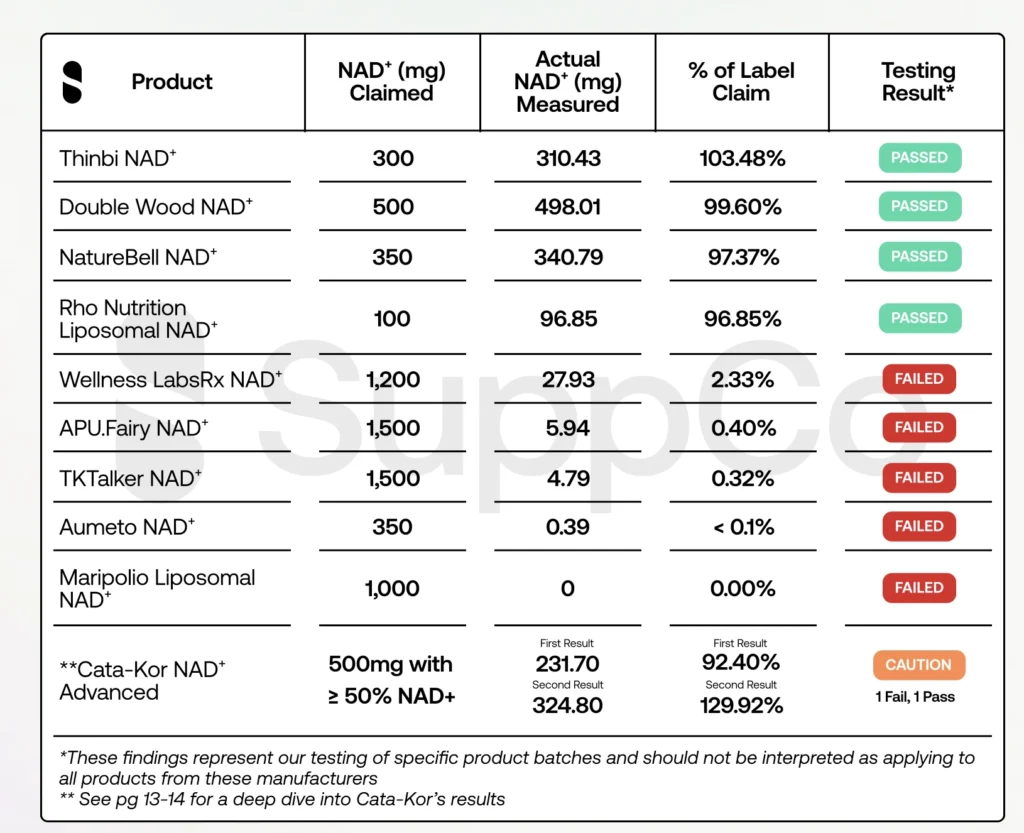
On a positive note, four of the products SuppCo tested passed with flying colors: Thinbi, NatureBell, Double Wood and Rho Nutrition. Thinbi, in particular, exceeding their label claims with 103% of the listed NAD+ present.

Another key takeaway from SuppCo? Watch for excessive dosage claims.
There’s no officially established daily dose for NAD+, but emerging research and most credible brands suggest serving sizes up to 500 mg. In SuppCo’s testing, many of the products that failed were the ones advertising far higher amounts. If an NAD+ supplement is promising an unusually high dose at a bargain price, SuppCo advises it’s worth a closer look. In many cases, sticking with a realistic, well-formulated serving may offer more reliable quality.
Industry Experts See a Broader Problem
Dr. Andrew Shao, senior vice president of global scientific and regulatory affairs at Niagen Bioscience (formerly ChromaDex) and the company behind NAD+ precursor Niagen, said the SuppCo results mirror what his team has seen in its own market surveillance testing.
He also argues that the supplement industry as a whole isn’t doing enough to ensure accountability and accurate labeling for NAD+ products.
“We believe that self-regulation is the path to responsible innovation,” Dr. Shao said. “Necessary changes must be made to truly protect consumers, including policy updates, enhanced enforcement and a collective industry-wide commitment to providing high-quality, science-backed products,” he said. “Until then, it’s up to each of us to make informed choices and demand better from the brands we trust.”
Although Niagen Bioscience holds what Shao describes as a robust patent portfolio on NR, sold as Niagen, the company frequently sees infringing products entering the market.

“In addition to the analyses we conducted on NAD+ and NMN products, we’ve also tested NR and counterfeit Tru Niagen products,” Dr. Shao said, adding that one report shows that 87% of tested NR supplements failed to meet their label claims, with many delivering only trace amounts (or none at all) of the active ingredient.
Dr. Shao emphasized that scientific research should be the foundation for supplement claims.
“One of the challenges is that dietary supplements in the U.S. are not subject to the same pre-market approval process as pharmaceuticals,” he said. “Without mandatory third-party testing or robust regulatory enforcement, low-quality, misleading products can enter and persist in the market unchecked. This not only risks wasting consumers’ money—it may erode trust in the entire supplement category.”
In the meantime, Dr. Shao’s advice to consumers begins with looking for a clearly labeled NAD+ precursor (Niagen NR) and independent, peer-reviewed published clinical studies as a key sign of credibility. Third-party testing certifications such as NSF, Alkemist Assured, or USP, along with FDA Generally Recognized as Safe (GRAS) and New Dietary Ingredient (NDI) notifications, also provide an extra level of assurance.
While the FDA does not regulate supplements in the same way as pharmaceuticals, Dr. Shao notes that few companies complete the required notification process. He further advises seeking products with regulatory authorization from bodies like the U.S. FDA or European Commission, avoiding NAD+ supplements that list NAD+ itself or NMN, and being cautious of “trendy delivery formats” that lack clinical support.